Creating Values to formulation

Supports & Services
With the help of expertise Sales team and Technical team Basil Group undertakes full exclusivity in Indenting, manufacturing and finished dosage formulations. Our skills coupled with years of experiences had been rendering value in terms of quality enrich products and timely deliveries to meet customer satisfactions as whole.
Adhering to high standard of ethics and integrity Basil Group have given glimpse of its presence, further supporting the pharmaceutical domain through several services.
Sales And Marketing Services
- Exclusivity – Rendering services through Exclusive tie-up with our principle companies, allows us to drive the trusted solutions in multiple workflow across the organisations.
- Market Intelligence – Our integrated market intelligence provide essential insights to our principle companies, delivering them the comprehensive global data as and when required.
- Focus on Sustainability – We believe in transition of commitments into action with data and analytics for sustainable future.
- Market Volatility – Measure, Manage and monitor the impact of turmoil market conditions.
- New product Developments – Expand your perspective by uncovering the essential insights in the market, further shaping the future of industries.

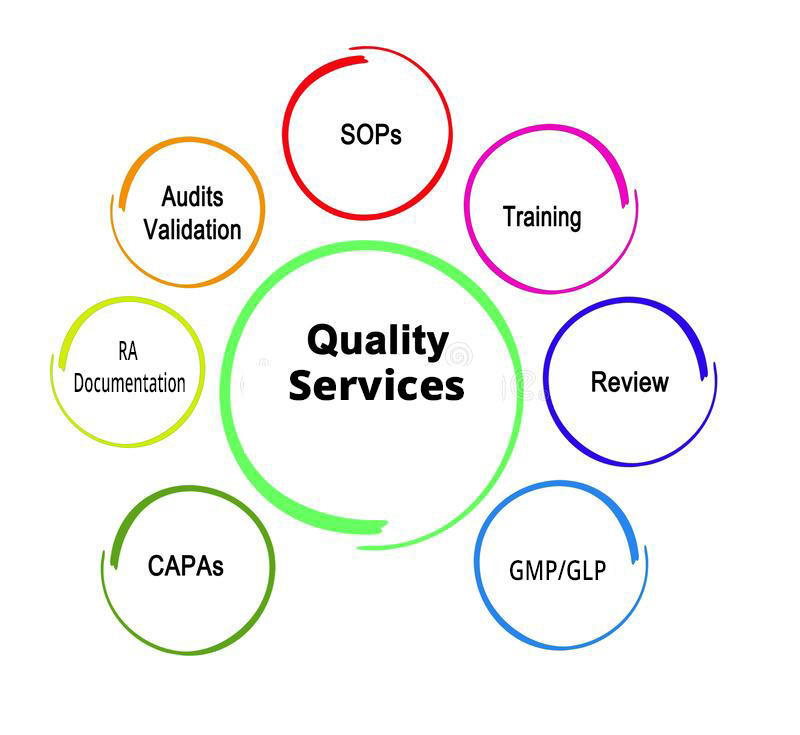
Quality Services
- Consultancy – Our most eligible team of quality professionals provide our client support services for QA/RA workings, inspections /Audit preparations, raw data handling, inspection remediation as per GMP/GLP standards.
- Simplified QMS Processes – Termed as Quality Management System, is a formalized business practice that defines management responsibilities for organizational structures, processes, procedures, and the resources needed to fulfill product and service requirements, customer satisfaction, and continuous improvement.
- Inspection and Audit Findings – Our team of professional auditors are committed towards systematic and independent examination, that brings up the activity to perform in organisation as per regulatory standards and also determined to establish the procedures that are effectively implemented to achieve required objectives.
Technical Services
- Manufacturing – We take this as our moral responsibility for services that includes a variety of activities ranging from Site identification, process Transfers to manufacturing on a commercial scale.
- Quality Control – Our capabilities include centres of excellence for FG and RM analysis, method development and validation, stability studies, impurity testing, cGMP quality control testing and cGMP batch release testing.
- Process Improvement – Our Services in process mapping is structured, through strategic process improvement with measurable incremental changes.
- New Product Development – Our success factor in this area of sustainability have been, the innovative culture, proper marketing support, qualified teams, proper internal and external relations and communications.
Partnerships







Head Office
A 609/610, Sagar Tech Plaza, Saki Naka Junction, Andheri – Kurla Road, Andheri East, Mumbai. Maharashtra, India. PIN – 400072.
Call Us
(+91) 022 4970 0250 / 0251 / 0252
Email Us
backoffice@basilpharmaceutical.com
info@basilpharmaceutical.com
procurement@basilpharmaceutical.com
fdf@basilpharmaceutical.com
